The Office of Academic Research (OAR) has prepared this training manual to guide investigators through the entire lifecycle of sponsored research, from pre-award (conceptualizing and submitting proposals) to post-award (managing the award, ensuring compliance, and closing out the project). This outline provides a step-by-step structure, highlighting critical requirements, regulations and resources.
Overview of Sponsored Research
Externally funded research programs are research initiatives that receive financial support from sources outside the host institution. These sources may include: federal agencies (e.g., NSF, NIH, DOE); state governments; industry partners; nonprofit organizations; or foundations or international sponsors. These funds are typically awarded through competitive proposals submitted by faculty or researchers (called Principal Investigators or PIs). Different types of awards have different rules and regulations; such as grants, cooperative agreements, contracts, subawards, etc.
Why is the PI handbook an important resource?
- It details the process for successful proposal development and submission. The handbook explains our processes and resources for the initial stages: grant development, approvals and submission.
- It clarifies PI eligibility, responsibilities and expectations. It outlines the PI’s role in managing the full lifecycle of a project—from proposal development to closeout. This includes resources for, project oversight, resource management, reporting, compliance and ethical conduct, and fiscal accountability.
- It ensures compliance with federal, state, and institutional regulations: The handbook helps PIs understand and follow complex regulations (e.g., Uniform Guidance, IRB, IACUC, export controls). Noncompliance can lead to audits, penalties, or loss of funding.
Suggested Uses of the PI Handbook
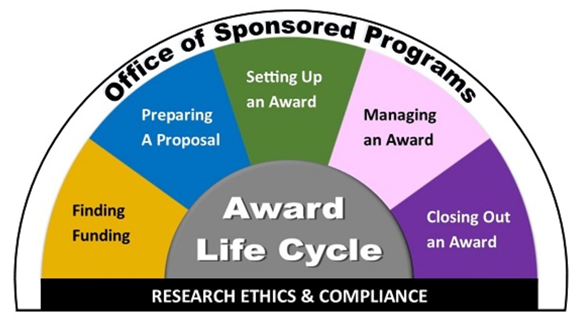
The Life Cycle of an Award
The lifecycle of a grant award encompasses the full journey of a funded project, beginning long before funds are received and continuing well beyond the final report. It typically starts with the identification of a funding opportunity and the development of a competitive proposal that aligns with the sponsor’s goals and guidelines. Once submitted and awarded, the pre-award phase transitions into post-award administration, where funds are managed according to federal, institutional, and sponsor-specific regulations. This phase includes project implementation, monitoring progress, managing budgets, and ensuring compliance with reporting and ethical standards. As the project concludes, the closeout phase involves submitting final technical and financial reports, documenting outcomes, and evaluating impact. Understanding this lifecycle is essential for faculty and research staff to ensure successful stewardship of external funding and maximize the potential for future grant success.
PI Eligibility
To be eligible, a PI must be a full-time permanent employee of CSUSB or the University Enterprises Corporation (UEC), such as tenure/tenure-track faculty, academic administrators, or center directors. Exceptions require prior administrative approval via the PI Approval Form. Students may not serve as PIs but can be co-investigators if sponsored by an eligible faculty or administrator. Visiting faculty may be listed as co-PIs under similar conditions. If a PI discontinues their role, Sponsored Programs Administration (SPA) must be notified, and a qualified replacement must be approved internally and by the sponsor. For more information see: “Eligibility to Serve as a Principal Investigator”.
PI Approval Form
Policy on Eligibility to Serve as a Principal Investigator
A Brief Overview of Roles and Responsibility of PIs
- Proposal Development. The PI Develops the technical narrative, goals, methodology and other required written components of the proposal. With the help of the Office of Research and Sponsored Programs (OSRP), the PI prepares or oversees budget and budget justification (ensuring costs are allowable and allocable) and meets submission requirements and deadlines.
- Project Leadership and Compliance: The PI initiates and leads the research or sponsored activity, ensuring alignment with the proposal’s scope of work. The PI provides technical direction and makes critical decisions related to the scientific or scholarly aspects of the project. The PI is responsible for Compliance and Risk Management including human subjects, animal research, and safety protocols.
- Fiscal Responsibility: The PI manages project funds in accordance with sponsor terms and conditions, university policies, Federal Uniform Guidance (2 CFR 200). The PI reviews expenditures regularly and works with the Office of Sponsored Programs Administration (SPA) to ensure budget accuracy and compliance.
- Personnel and Effort Oversight. As required, the PI recruits, supervises, and evaluates project staff, students, collaborators, or project sub awardees. The PI ensures that personnel effort commitments are met and accurately reported in a timely and truthful manner.
- Communication and Collaboration. The PI maintains clear communication with SPA, relevant campus departments, and the funding agency. The PI notifies SPA of significant changes in project scope, personnel, or budget, as is needed to ensure agency compliance.
Project Reporting and Closeout. The PI submits technical and programmatic reports on time. Participates in closeout procedures, including: final financial review; submission of final reports; ensuring project assets and data are handled appropriately.